A closer look at Canada’s homegrown COVID-19 vaccine candidates
[ad_1]
More than 100 groups around the world are racing to produce a vaccine against COVID-19, with most of the attention focused on front-runners currently in Phase 3 clinical trials in the U.S. and overseas.
But Canada has also invested in some COVID-19 vaccines in development here, and there are more than half a dozen Canadian vaccine candidates registered with the World Health Organization, at least one of which is already being tested on humans. They represent a wide range of technologies, from more traditional protein subunit vaccines to newer technologies such as replicating viral vector and DNA vaccines. The options, if approved, would include both needles and a nasal spray.
Dr. Volker Gerdts, director and CEO of the Vaccine and Infectious Disease Organization-International Vaccine Centre at the University of Saskatchewan in Saskatoon, argues that “it’s very important … to be self-sufficient and have access to vaccines that are being produced here in Canada for Canadians.”
He and other advocates say that will give Canadians more control over when and how vaccines become available here.
The federal government recently invested $1 billion dollars in preorders for six foreign vaccine candidates, even though there’s no guarantee that any of them will ever make it through clinical trials to market.
But some Canadian vaccine developers have reported facing big hurdles in development, including not enough government support. Gerdts said lack of manufacturing capacity in Canada slowed efforts earlier this summer.
Michael Houghton, who is leading a vaccine development team at the University of Alberta, said lack of funding to manufacture vaccines for a clinical trial has set his team back. Providence Therapeutics, a Toronto-based company whose mRNA vaccine is not listed with the WHO, has complained about a lack of government support for clinical trials. However, some teams, such as Halifax-based IMV and Edmonton-based Entos, have announced getting federal government funding to proceed with trials.

Stephen Barr, who is leading a vaccine development team at Western University, says it’s important to support the development of multiple vaccine candidates, as some may be better for certain populations than others.
“The best candidate may not be best for everybody,” he said. Some may have other advantages, such as being cheaper to produce or logistically easier to store or ship, he added.
Despite the challenges, many Canadian researchers are plugging away at a variety of technologies and strategies. Here’s a closer look at the COVID-19 vaccines produced by Canadian teams listed by the World Health Organization.
WATCH | How wealthy countries buying up vaccine supplies could hinder pandemic fight:
Vaccine nationalism, when rich countries buy up vaccines making them unavailable for other countries, could hinder the global fight to end the COVID-19 pandemic and a program to have vaccines available everywhere is still not fully funded. 4:12
Entos Pharmaceuticals
Location: Edmonton
Vaccine type: DNA
Stage of development: Preclinical
Entos Pharmaceuticals is a University of Alberta spinoff focused on genetic therapies.
It’s working on a DNA-based vaccine against COVID-19 that will work by delivering genes from SARS-CoV-2 into the body. The body’s cells then use those instructions to make coronavirus proteins, exposing the immune system to them so it can learn to recognize and fight off SARS-CoV-2.
It’s similar to gene-based RNA vaccines like the ones being made by Moderna and Pfizer/BioNTech, with some tradeoffs. DNA is more stable than RNA, which means it can be stored and shipped more easily, and it stays active in the body for longer. But DNA needs to get into the nucleus of the cells in the body, and it’s more complicated to deliver effectively.
Entos’s key technology is a way of delivering the DNA. DNA and RNA are typically packed into tiny spheres called lipid nanoparticles for delivery into the body. Normally, those are engulfed by cells whole, which means that once inside the cell, the DNA or RNA still has to escape its container, said John Lewis, CEO of Entos Pharmaceuticals and an associate professor at the University of Alberta.
Entos’s Fusogenix technology, on the other hand, is a “fusion protein” on the outside of the nanoparticle that fuses the nanoparticles with human cell membranes, freeing the DNA from the nanoparticle as it enters the cell.
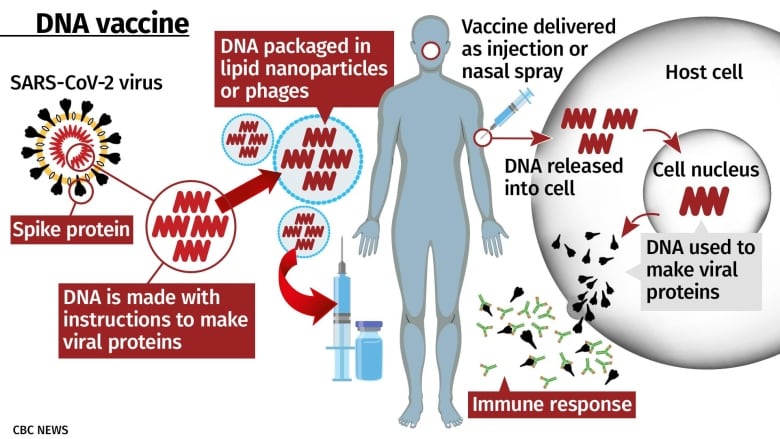
The DNA in Entos’s vaccine contains genes for both the SARS-CoV-2 spike protein and the n-protein, a protein that is similar among different coronaviruses.
That means it could potentially provide protection against other coronaviruses, Lewis said.
The DNA also contains two “genetic adjuvants,” special codes designed to enhance the immune response to the vaccine.
Once the DNA has entered the nucleus of human cells, the instructions are used to make the coronavirus proteins, which are displayed on the cell surface and released into the bloodstream to generate an immune response.
Lewis said animal tests so far suggest a single dose will be enough to generate good immunity, making it logistically easier to deliver than a vaccine requiring two doses.
As of early October, Entos was aiming to start Phase 1 clinical trials at the Canadian Centre for Vaccinology in Halifax in November. Lewis said the company had also received funding to proceed to the end of Phase 2 trials.
WATCH | How COVID-19 vaccines are being created in months, rather than years:
Some potential COVID-19 vaccines are already in the third stage of clinical trials. It’s taken a lot of effort and money to squeeze a process that can normally take five years into about 10 months and still be done safely. 2:17
IMV
Location: Halifax
Vaccine type: Protein subunit
Stage of development: Preclinical
IMV’s vaccine is a protein-based vaccine, a relatively traditional and widely used vaccine type, where pieces of viral protein are injected to teach the immune system to recognize them. Like many teams around the world (including Novavax, which is in Phase 3 clinical trials), IMV is focused on the SARS-CoV-2 spike protein.
“But instead of giving the immune system the entire spike protein, we’ve selected very small regions that have been described to be important for its function,” said Marianne Stanford, the company’s vice-president of research and development.
In IMV’s case, the pieces are so small that they’re pieces of protein called peptides, which don’t need to be manufactured by living organisms — simple chemistry is all you need.
“Many manufacturers all over the world can make peptides in pretty significant quantities,” Stanford said. “And the fact that our our whole vaccine is synthetic is an advantage because we can scale it up reasonably simply.”

The vaccine consists of four peptides from the spike protein. Instead of putting them in a water-based solution like many other vaccines, IMV uses an oil “which holds it at the site of injection,” Stanford said. The idea is that concentrates it at the site of injection instead of dispersing it through the body, which allows the immune system to interact with it over a longer period of time and generate a stronger response from a small dose.
The vaccine has been tested on mice and ferrets. On Oct. 8, the company said it had received additional funding and support from the federal government, including $5.4 million for clinical trials. IMV says it plans to start a combined Phase 1/2 clinical trial with the Canadian Centre for Vaccinology in Halifax after more preclinical safety studies, but did not say when. It also said it was collaborating with a “global manufacturing partner” with facilities in India and Europe to scale up production of the vaccine to several hundred million doses if it’s approved.
WATCH | Canada’s chief public health officer discusses Health Canada’s vaccine approval process:
Dr. Theresa Tam, Canada’s chief public health officer, sidestepped a question about a new Russian coronavirus vaccine, saying Canada uses ‘solid’ processes to ensure the quality and safety of its vaccines. 1:29
Medicago
Location: Quebec City
Vaccine type: VLP (virus-like particle)
Stage of development: Phase 1 clinical trials
Medicago is a Canadian subsidiary of Japan’s Mitsubishi Tanabe Pharma Corp.
Its COVID-19 vaccine candidate became the first in Canada to start human trials in July.
The main component of the vaccine is the spike-protein from SARS-CoV-2, the coronavirus that causes COVID-19, but multiple spike proteins are assembled into a virus-like particle, or VLP.
“The virus-like particle has the advantage of looking like a virus to the immune system without being infective,” said Nathalie Landry, the company’s executive vice-president of scientific and medical affairs. That generates a good immune response, she said.

The particles are made by inserting the spike protein gene into plants — tobacco relatives called Nicotiana. The plant produces the protein for about a week, automatically assembling the proteins into microscopic spheres that consist of membranes studded with spike protein.
The leaves are then crushed and the VLPs are purified, Landry said.
The protein is easier to scale up and purify when grown in plants than in animal cells, as some other teams around the world are doing. The vaccine can be stored in an ordinary refrigerator. Landry said there are also some indications it may be stable at room temperature, making it easier to store and distribute than other vaccines that require extremely cold temperatures, such as -20 C or -80 C for RNA vaccines.
Tests on mice showed a positive immune response 10 days after a single dose, the company said in a news release. The results have not be published in a peer-reviewed journal, but Medicago says it plans to announce Phase 1 human trial results in October and publish its results from both mouse and monkey trials after that.

During human tests, the company is combining the VLPs with proprietary adjuvants from Dynavax and GSK in an effort to enhance the immune response to the vaccine and decrease the amount of vaccine needed per dose.
The company has been working for 20 years on the vaccine technology, which was originally developed by Agri-Food Canada and Laval University. It’s previously been used to create a seasonal flu vaccine that is being reviewed by Health Canada, and, if approved, would be the first plant-based vaccine in the world, the company says.
For the COVID-19 vaccine, the company has received federal funding for both animal and human testing, as well as for expansion of its manufacturing capacity. The Quebec government also gave Medicago $7 million.
Landry said the company expects to be able to produce 20 million doses a year at its plant in Quebec, and another 100 million doses a year at its plant in Raleigh, N.C. However, CEO Bruce Clark has previously suggested the U.S. location of that plant means there’s no guarantee of a Canadian supply of the vaccine if it’s successful.
WATCH | Quebec company begins human trials for potential COVID-19 vaccine:
Medicago says it administered the first doses of the coronavirus vaccine candidate to volunteers this week. The trial is a study of 180 healthy men and women between 18 and 55 years old. 7:49
Mediphage Bioceuticals/University of Waterloo
Location: Toronto/Waterloo, Ont.
Vaccine type: DNA
Stage of development: Preclinical
If you don’t like needles, you may be interested in the COVID-19 vaccine being developed at the University of Waterloo. It’s being designed for administration as a nasal spray.
Roderick Slavcev, chief scientific officer at Mediphage Bioceuticals Inc. and an associate professor of pharmacy at the University of Waterloo whose specialities include vaccine design, said the goal is to mimic the route of infection that SARS-CoV-2 normally takes, including targeting the right cells in the lungs and lower respiratory tract.
“There’s good data that suggests that by doing so, you generate the most pertinent type of [immune] response,” he said.
The downside is that it doesn’t work if your nose is congested.
The vaccine itself is a DNA vaccine. Instead of containing a virus or viral protein, it contains only the genetic instructions for making one or more proteins. Once it gets inside the body, human cells will make viral proteins based on the instructions.
One of the challenges with DNA vaccines is how to get the DNA into cells. The Waterloo team is using to possible strategies:
-
Putting the DNA into a liposomal nanoparticle, similar the ones used in similar RNA vaccines such as Moderna.
-
Packaging the DNA into a bacteriophage, a virus that only infects bacteria, using technology from Toronto-based University of Waterloo spinoff Mediphage Bioceuticals.
Using bacteriophages means the team can infect bacterial cells in the lab to produce “massive amounts” of the bacteriophage and the DNA, making it easy to scale up production. While phages can’t infect human cells, the ones in the vaccine have been fused to peptides — protein subunits — that bind to the ACE-2 receptor in human cells. That’s the same receptor that binds to the coronavirus spike protein and lets it enter cells.
Once that happens, Slavcev says, the phage enters the cell, gets broken down, and releases its DNA.
The DNA contains not just instructions for the spike protein in this case, but also the protein that forms the outer membrane or “envelope” of the coronavirus. The system is designed to generate not just spike proteins, but an entire “virus-like particles,” or VLPs.
“You’re forming something that looks almost entirely like the virus, but has no genetic material,” Slavcev said. Each cell can generate large quantities of VLPs for up to two weeks (a far longer effect than for an RNA vaccine) that can leave the cell and trigger a broader immune response than viral proteins alone. The phage itself also generates an immune response, acting as an adjuvant.
Slavcev said on Oct. 9 that the team expected to start preclinical (animal) trials later in the month.
University of Alberta
Location: Edmonton
Vaccine type: Protein subunit
Stage of development: Preclinical
The University of Alberta’s vaccine development team is led by Prof. Michael Houghton, director of the Li Ka Shing Applied Virology Institute and recent co-winner of the Nobel Prize in Medicine.
It’s a protein subunit vaccine, which Houghton describes as “very well tried and very well tested” technology compared to mRNA, DNA and viral vector vaccines.
In this case, a SARS-CoV-2 gene is inserted into mammal cells to produce large quantities of a viral protein that’s subsequently injected in the body as a vaccine. Like IMV, the University of Alberta isn’t targeting the entire SARS-CoV-2 spike protein, just a small piece of it — the receptor binding domain (RBD), the part that binds to human cells in order to enter them.
Houghton said that region activates the production of at least three kinds of neutralizing antibodies that can stop an infection. And it can be produced much more efficiently than the entire spike protein.
He said that it can likely be purified more easily than an entire protein and reduces the risk of antibody-dependent enhancement, a potential problem where “non-neutralizing”antibodies are produced and end up enhancing infection instead of neutralizing it.
The vaccine would ultimately contain both the RBD protein and a commercial adjuvant, as is typical for protein-based vaccines.
WATCH | Will life return to normal once a COVID-19 vaccine is available?
An epidemiologist and infectious disease specialist answer questions about a COVID-19 vaccine, including what happens after a vaccine is approved and available in Canada. 4:54
As of early October, Houghton said the team had done successful preclinical tests and was trying to get funding for manufacturing and clinical testing, but didn’t have it yet, after failing to obtain a federal grant. The team is currently preparing a “clinical-grade” cell line and has partnered with an adjuvant maker to be prepared for clinical testing anyway.
Houghton noted that the international vaccine frontrunners are currently in Phase 3 clinical trials, but mostly represent newer technologies.
“We will be ready with our tried and tested adjuvanted protein platform just in case the Phase 3 trials disappoint (which will be very alarming),” he wrote in an email.
However, even if those trials succeed, he said a protein subunit vaccine may be used as a booster to promote long-term immunity, as the newer vaccine types will likely be more expensive and have only been tested with two shots.
“We do not know if they will be well-tolerated after three shots,” he said.
WATCH | Who will be on the priority list in Canada to receive a vaccine first?
When the first COVID-19 vaccines become available, there won’t be enough for everyone who wants it. So, which Canadians should get it first and how will that be decided? Dr. Peter Lin answers that and other questions from viewers. 7:22
University of Manitoba
Location: Winnipeg
Vaccine types: VLP, replicating viral vector
Stage of development: Preclinical
Dr. Xiao-Jian Yao, a professor of medical biology at the University of Manitoba, is leading the development of two COVID-19 vaccine candidates.
VLP vaccine
Like Medicago, Yao and his team put coronavirus genes into other cells to produce coronavirus proteins in the form of virus-like particles.
In this case, the spherical particles are grown in mammalian cells and studded with two proteins:
-
The receptor binding domain (RBD) of the SARS-CoV-2 spike protein — the subunit of the spike protein that actually attaches to human cells, allowing the virus to enter.
-
An Ebola virus protein that targets special immune cells called dendritic cells to generate a stronger immune response.
Yao and his team are currently testing this vaccine in mice and trying to produce it more efficiently.
Replicating viral vector vaccine
Viral vector vaccines against COVID-19 use a “carrier” virus to bring coronavirus genes — and therefore a coronavirus protein itself — into the human body.
Using viral vectors is a strategy used by many teams developing a COVID-19 vaccine around the world, including three in Phase 3 clinical trials: University of Oxford/AstraZeneca; Janssen, a subsidiary of Johnson & Johnson; and Russia’s Gamaleya Research Institute.
The University of Manitoba team is using a viral vector called the vesicular stomatitis virus (VSV), which mainly infects livestock such as horses and pigs. Humans aren’t typically exposed to it unless they work with animals, generally don’t show symptoms if infected, and can’t transmit it to other humans.
It’s similar to the system (also based on VSV) used to make the Canadian-developed Ervebo Ebola vaccine, which has been approved by U.S. and European regulators. That vaccine has been used to vaccinate hundreds of thousands of people in Congo.
Unlike the adenoviruses used in the vaccines currently in Phase 3 trials, the VSV vector can replicate in the body and only a small amount is needed for each dose.
Yao’s team is putting into the VSV vector the same two proteins that they’re targeting in the VLP vaccine.
At the moment, they’re still working on the last steps of the method for producing the vaccine.

VIDO-Intervac
Location: Saskatoon, Sask.
Vaccine type: Protein subunit
Stage of development: Preclinical
The University of Saskatchewan’s Vaccine and Infectious Disease Organization-International Vaccine Centre (VIDO-Intervac) has previously produced two coronavirus vaccines — one for cattle and one for pigs.
Just before the COVID-19 pandemic hit, it was working on a vaccine against the MERS coronavirus.
“In essence, it’s almost identical to the approach we’re using right now,” said Dr. Volker Gerdts, director and CEO of VIDO-Intervac.
The vaccine is a protein subunit vaccine made up of molecules of the virus’s spike protein. It’s made by putting the gene for the protein into a culture of mammalian cells, which instructs them on how to make the protein. It’s a vaccine approach that many groups around the world are taking.
“But what is unique about our vaccine is we’re mixing this protein with an adjuvant…that really now drives the immune response toward a certain direction,” Gerdts said.
WATCH | Canada’s role in the global race to find a COVID-19 vaccine:

Canada’s leading vaccine researcher and a team at the University of Saskatchewan are working around the clock to find a COVID-19 vaccine to save lives. 3:04
Vaccines made from proteins instead of entire viruses is generally don’t activate all arms of the immune system. Adjuvants are extra compounds intended to compensate for that.
“So they mimic, essentially, a full-blown infection and provide what we call the ‘danger signal’ to the immune system,” Gerdts said. “I would argue the adjuvant in the vaccine is almost as important as the actual protein.”
The adjuvant VIDO-Intervac is testing uses three different chemical compounds to convey different kinds of “danger signals.” In doing so, it activates immune cells called T-cells.
“That is something that, in addition to neutralizing antibodies, we seem to need,” Gerdts said.
In May, the researchers announced their vaccine was “highly effective” in preclinical trials in ferrets, generating antibodies and decreasing viral infection. However, before moving to human trials, the researchers need to complete studies using higher-grade materials, and production was delayed by busy manufacturers, they reported in August. However, as of early October, the Gerdts said the materials had been manufactured, the toxicology studies were nearly complete and his team hoped to start clinical trials at the Canadian Centre for Vaccinology in Halifax in December.
Western University
Location: London, Ont.
Vaccine type: Replicating viral vector
Stage of development: Preclinical
In March, vaccine researchers at Western University in London, Ont., were just starting Phase 1 and Phase 2 clinical trials for a vaccine against the MERS coronavirus.
When the COVID-19 pandemic hit Canada that month, the trials were put on hold. But the researchers didn’t stop working — they just switched to SARS-CoV-2 instead.
The system they had been working on was a replicating viral vector vaccine, using the same viral vector as the University of Manitoba, the vesicular stomatitis virus (VSV).
Stephen Barr, an associate professor at Western University’s Schulich School of Medicine and Dentistry and co-leader of the vaccine team, said when the coronavirus spike protein gene is inserted into VSV, the virus develops a coat around it that looks like SARS-CoV-2, which teaches the immune system to recognize it.
“You don’t need to inject a lot of the virus into the body because it can make copies of itself,” Barr said. That could make it quicker and cheaper to produce than vaccines based on non-replicating viruses. “And also because it can make copies of itself, it mimics more what a natural virus would do. It would go through that whole process of finding a cell, getting into a cell, making proteins.”
The technology, unlike some others, has already been commercialized and shown to work for the Ebola vaccine — “which is why we think it will have a good chance of success,” Barr said.
As of early October, Barr said two versions of the COVID-19 vaccine had generated good antibody responses in animal tests. The team is trying to get funding to proceed to combined Phase 1/2 clinical trials.

[ad_2]
SOURCE NEWS
